Low molecular weight polysialic acid as glycome-based biologic for the therapy of dry age-related macular degeneration
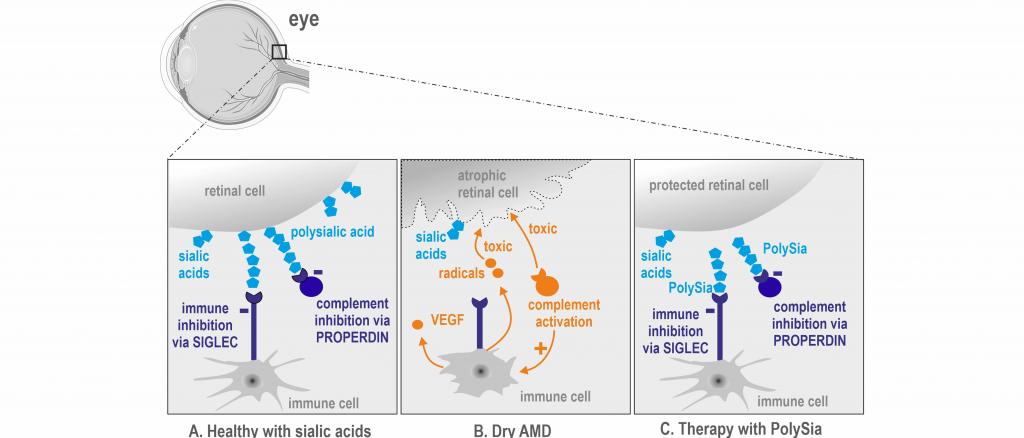
Increased activity of the innate immune system is an essential pathophysiological aspect in the progression of age-related macular degeneration (AMD). Both cellular processes of invading or locally activated mononuclear phagocytes (immune cells) of the retina and a hyperactive complement system (soluble immune proteins of the plasma) contribute to the degeneration of nerve cells in the retina. Especially the macula, the area of our sharpest vision in the retina, is affected in dry AMD. Currently, there is no approved therapy for the treatment of dry AMD. Inhibition of the alternative complement activation is being pursued as a possible therapeutic approach in clinical studies.
Polysialic acids have an inhibiting effect on human mononuclear phagocytes and microglial cells via SIGLEC-11 receptors. By binding of polysialic acids to the SIGLEC-11 receptors of the mononuclear phagocytes, release of radicals, inflammatory cytokines and the growth factor VEGF is suppressed. Furthermore, polysialic acids interact with the complement activator PROPERDIN and thus prevent activation of the alternative complement signaling pathway. The intravitreal administration of 0. 2 µg polysialic acid in the SIGLEC-11-transgenic mouse model after a laser damage-induced lesion (AMD-like mouse model) showed an inhibiting therapeutic effect on the mononuclear phagocytes and microglial cells of the retina, on complement activation and also on vascular leakage. In this way, polysialic acids prevent the typical immune derailments of AMD (radical production, inflammatory reaction, VEGF release, vascular leakage and complement activation) and help to rebuild the natural balance in the retina.
This project aims to advance the preclinical development of a low molecular weight polysialic acid (PolySia) therapy for AMD. For this purpose, the basic clinical applicability of the established polysialic acid production process should be confirmed and the production of high quality polysialic acid should be carried out for the planned experiments. In addition, an optimized formulation of the polysialic acid for intravitreal administration will be established. The bioassay and bioanalytics of polysialic acids will be improved and validated. In addition, an acute GLP-based preclinical safety pharmacology of polysialic acid is planned to be carried out in humanized SIGLEC-11 transgenic mice.
Here you can find further information.