Drug substance production of the bispecific PSMAxCD3 antibody CC-1 to enable the conduct of a clinical study in patients with squamous cell carcinoma of the lung
Applicant: Prof. Dr. med. Helmut Salih, Universitätsklinikum Tübingen
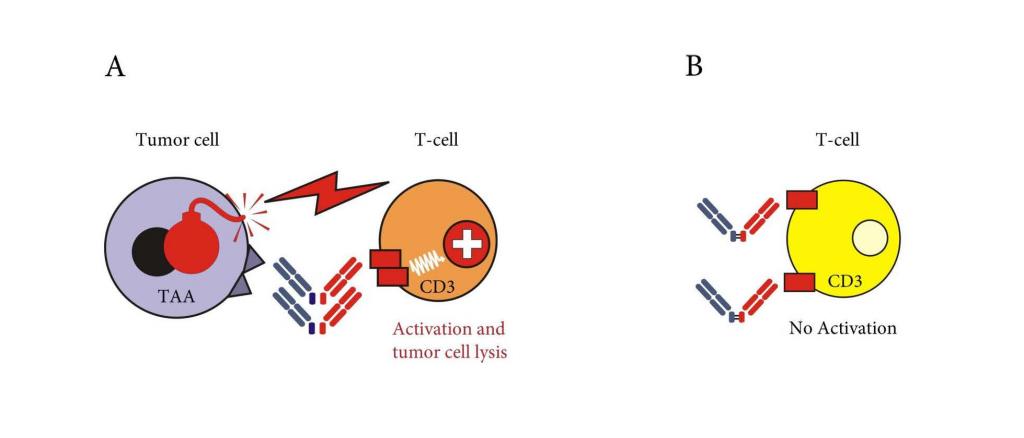
CC-1 is a bispecific antibody (bsAb) with PSMAxCD3 specificity developed within the German Cancer Consortium (DKTK). Besides prototypical binding to prostate cancer cells, CC-1 additionally reacts with squamous cell carcinoma cells of the lung. Furthermore, CC-1 binds to tumor vessels, thereby allowing for a dual mode of anti-cancer action. CC-1 was developed in a novel format which not only prolongs serum half-life, but most importantly reduces off-target T cell activation with fewer expected side effects. Together with preemptive IL-6 receptor blockade using Tocilizumab, this allows for application of effective bsAb doses with high anticancer activity. The regulatory authority has already approved a clinical study with CC-1 in prostate cancer which is meanwhile recruiting, and the agency also indicated general approval of our study concept in lung cancer.
Funding of this project by the ForTra gGmbH enables the required production of CC-1 drug substance under Good Manufacturing Practice (GMP) conditions, thereby allowing the conduct of a first clinical study with CC-1 in human lung carcinoma patients that will evaluate safety and efficacy of the treatment. Overall, our project fascilitates the evaluation of a novel immunotherapeutic strategy in a cancer entity with unmet medical need, and it bridges the gap between discovery of innovative biologicals and their clinical evaluation.
Here you can get further information.
Project partner:
Philogen S.p.A., Siena, Italy




