Targeting a Common Checkpoint of Brain Disease by novel Autotaxin inhibitors
Project partner:
Prof. Dr. med. Johannes Vogt, Direktor des Instituts für Neuroanatomie der Universität Köln, Prof. Dr. med. Timo Uphaus, EKFS-Tanslations-Professur an der Klinik für Neurologie der Universitätsmedizin Mainz, Prof. Dr. med. Dr. phil. Dipl.-Psych. Udo Dannlowski, Direktor des Instituts für Translationale Psychiatrie, Universitätsklinikum Münster
Project:
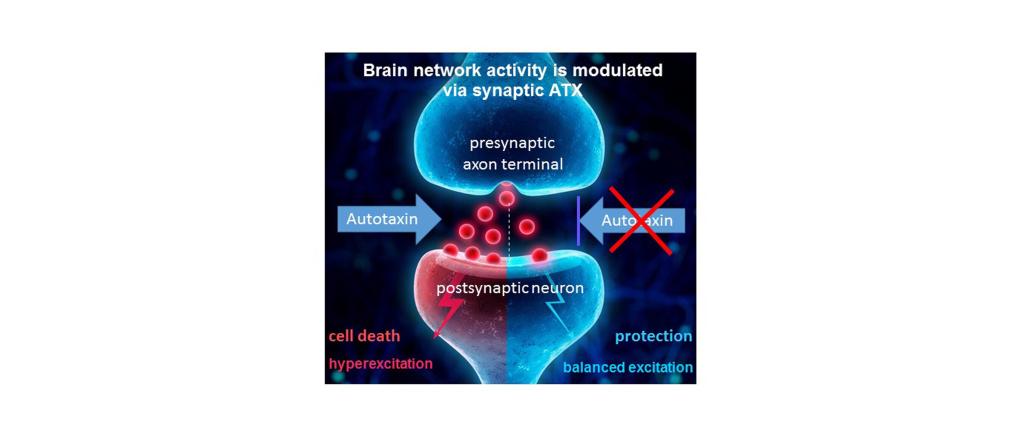
Prof. Dr. med. Dr. phil. Robert Nitsch and his team aim to transition an innovative approach to treat brain disorders for which there are no efficient drug therapies. Autotaxin is an enzyme which produces the bioactive lipid lysophosphatidic acid (LPA) at the cortical synapse. Novel brain-penetrating autotaxin inhibitors (ATX) shall help patients suffering from different neuropsychiatric disorders that share pathologic alterations at a common check-point: the synaptic excitation.
The 100 billion nerve cells in the brain are connected to each other with over a trillion synapses, the central checkpoint of brain function. A precise regulation of this checkpoint enables a balance between inhibition and excitation (E/I-balance) within the neuronal networks in the brain, which is a prerequisite for healthy brain function. Overexcitation at this checkpoint leads to dysfunction of the networks, and can also lead to the demise of neurons that build these networks.
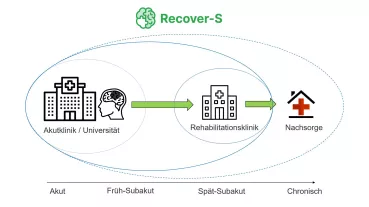
In different disease models, key markers for mental disorders known to be defined by an altered E/I-balance at the synaptic checkpoint were brought to normal levels by ATX-inhibition, with no evident side effects. Since ATX controls lipid signaling in the brain, fasting-induced hyperphagia was reduced upon ATX inhibition, and obese mice showed significant weight loss. Inhibition of ATX also resulted in balanced excitation after stroke, thereby reducing secondary tissue damage surrounding the infarct core. This led to a significantly improved stroke outcome in a mouse model even when treatmeht started hours after the initial ischemic event.
The current ForTra-supported translational project is designed to perform IND (Investigational New Drug Application) - enabling preclinical studies with the novel MJK ATX-inhibitors, as a prerequisite for authorization of a phase I clinical study by the Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) on safety, tolerability, and dose range.
Here you can find further information.