Phase I trial of the bispecific PSMAxCD3 antibody CC-1 in biochemical recurrence of prostate cancer
Project partner:
AKF (Angewandte Klinische Forschung) program of the University Hospital Tübingen
Project:
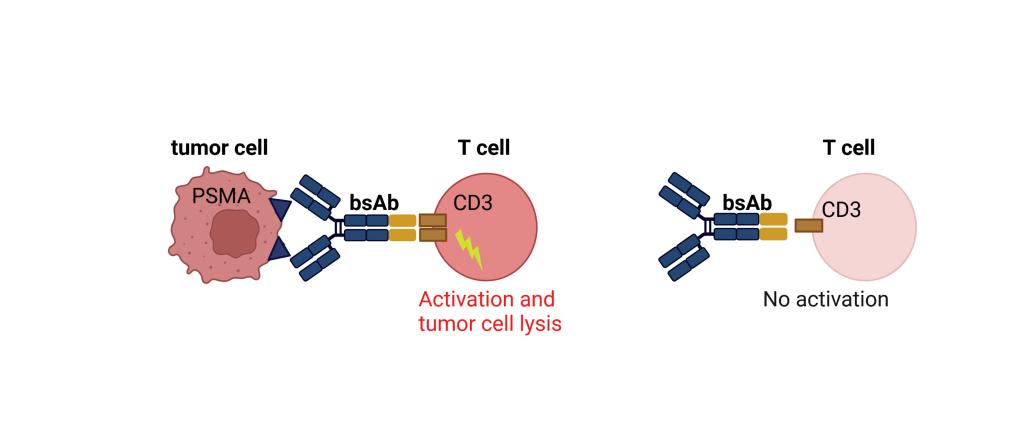
CC-1 is a bispecific antibody with PSMAxCD3 specificity developed within the German Consortium for Translational Cancer Research (DKTK). CC-1 binds to prostate cancer cells and additionally also to tumor vessels, enabling a dual mechanism of action. CC-1 was developed in a novel format that not only allows for prolonged serum half-life, but more importantly reduces unwanted "off-target" activation of T cells and thus side effects.
A first clinical study with CC-1 in patients with metastatic prostate carcinoma has already been conducted, and the antibody shows good tolerability and first signs of clinical efficacy. Now, with the support of ForTra gGmbH, a further study in men with biochemical recurrence of prostate carcinoma will start. With the clinical evaluation in the early disease situation, this new immunotherapy minimizing severe side effects will be brought to patients.
Here you can find further information.
Details about the study can be found here.