Omission of sentinel lymph node biopsy in triple-negative and HER2-positive breast cancer patients with radiologic and pathologic complete response in the breast after neoadjuvant systemic therapy: a single-arm, prospective surgical trial. EUBREAST-01 tri
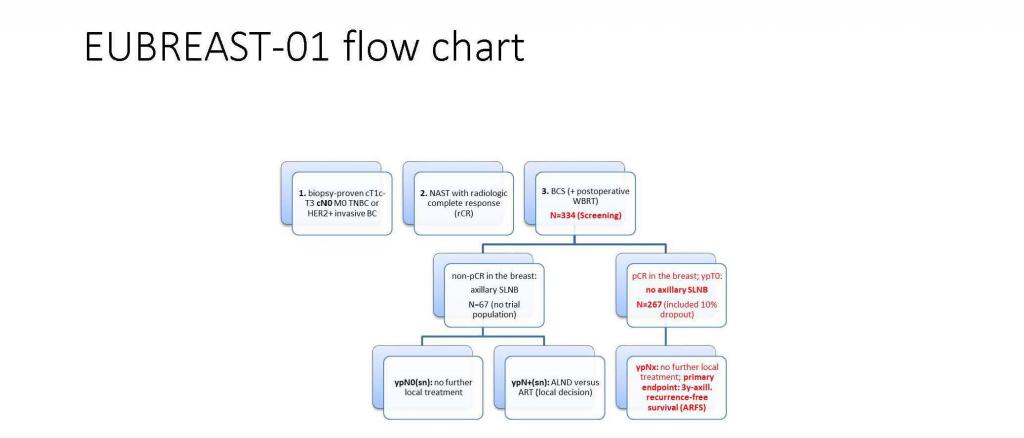
Improvements in systemic treatments for breast cancer have increased the rates of pathologic complete response (pCR) in patients receiving neoadjuvant systemic therapy (NAST), offering the opportunity to reduce surgery in patients who have a pCR. We include only patients with the highest likelihood of having a pCR after NAST (triple-negative or HER2-positive breast cancer). In the trial, axillary surgery will be eliminated completely for initially cN0 patients with radiologic complete remission and a breast pCR as determined in the lumpectomy specimen.
The trial design is a multicenter single-arm study with a limited number of patients (N=267). Patients will be recruited in European countries (Austria, Germany, Italy, Spain and Sweden) over a period of 24 months.
Here you can get further information.



